Background: Ide-cel, a BCMA-directed CAR T cell therapy, showed tolerability and promising efficacy in patients with relapsed and/or refractory multiple myeloma (RRMM) in the first-in-human phase 1 CRB-401 study (Raje et al. N Engl J Med. 2019;380:1726) and the pivotal phase 2 KarMMa study (Munshi et al. J Clin Oncol. 2020;38[suppl, abstr]:8503). Ide-cel demonstrated a favorable benefit-risk profile with an overall response rate (ORR) of 85%, a complete response (CR) rate of 45%, and a median progression-free survival (PFS) of 11.8 months in the first 33 patients treated in CRB-401. Reported here are updated safety and efficacy results for 62 patients who received ide-cel in the ongoing CRB-401 study.
Methods: CRB-401 (NCT02658929) is a 2-part, phase 1 dose-escalation and -expansion study. The expansion phase enrolled patients who had received ≥3 prior lines of therapy, including a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 antibody, and were refractory to their last line of therapy. Eligibility criteria for the dose-escalation phase were described previously (Raje et al. N Engl J Med. 2019;380:1726). After lymphodepletion with fludarabine (30 mg/m2/day) and cyclophosphamide (300 mg/m2/day) for 3 days followed by 2 days of rest, patients received ide-cel at target doses of 50, 150, 450, or 800 × 106 CAR+ T cells in the dose-escalation phase and 150 to 450 × 106 CAR+ T cells in the dose-expansion phase. The primary endpoint was safety. Secondary endpoints included tumor response according to the International Myeloma Working Group criteria. Exploratory endpoints included PFS, overall survival (OS), and minimal residual disease (MRD).
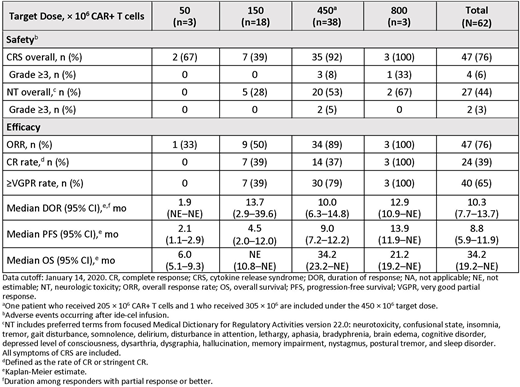
Results: As of January 14, 2020, 21 patients had received ide-cel in the dose-escalation phase, and 41 patients received ide-cel in the dose-expansion phase. The median age was 61 years, and 44% of patients had high tumor burden (≥50% bone marrow CD138+ plasma cells). Of the 62 patients, 45% received >6 prior regimens, 90% were daratumumab-exposed, and 77% were daratumumab-refractory. As of the cutoff date, 13 patients were ongoing, and 49 patients had discontinued the study. Reasons for study discontinuation were progressive disease (58%), withdrawal by patients (10%), and death (10%). Based on safety and efficacy in the dose-escalation phase, target dose levels of 150 to 450 × 106 CAR+ T cells were selected for the dose-expansion phase. The most frequent adverse events (AEs) were neutropenia (92%), cytokine release syndrome (CRS; 76%), anemia (76%), and thrombocytopenia (74%). The most frequent grade 3/4 AEs were neutropenia (89%), leukopenia (61%), anemia (57%), and thrombocytopenia (57%). Most CRS events were grade 1 or 2 (Table). Four patients (7%) had grade 3 CRS; there were no grade >3 CRS events. The incidence of CRS generally increased with target dose level. Neurologic toxicity (NT; clustered term) occurred in 27 patients (44%) and was primarily grade 1/2 with 1 patient having grade 3 and 1 patient having grade 4 NT. Among all 62 patients in the dose-escalation and -expansion phases, the ORR was 76%, including 24 patients (39%) with a CR or better and 40 patients (65%) with a very good partial response or better. The median duration of response was 10.3 months. Of 37 responders evaluable for MRD, 30 were MRD negative (≤10−4 nucleated cells) at 1 or more time point, and 7 responders were MRD positive. With a median follow-up of 14.7 months for all patients in the dose-escalation and dose-expansion phases, median PFS was 8.8 months and median OS was 34.2 months. Overall, a dose-dependent effect was observed on responses and survival outcomes, with greater efficacy reported at ≥150 × 106 CAR+ T cells (Table).
Conclusions: Ide-cel demonstrated deep and durable responses in heavily-pretreated RRMM patients. Efficacy and safety reflect prior reports and support a favorable clinical benefit-risk profile for ide-cel at target dose levels ≥150 × 106 CAR+ T cells.
Lin:Novartis: Consultancy; Janssen: Consultancy, Research Funding; Vineti: Consultancy; Sorrento: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gamida Cells: Consultancy; Takeda: Research Funding; Merck: Research Funding; Legend BioTech: Consultancy; Juno: Consultancy; Celgene: Consultancy, Research Funding; Bluebird Bio: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding. Raje:Caribou: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy; Bluebird, Bio: Consultancy, Research Funding; Takeda: Consultancy; Immuneel: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Karyopharm: Consultancy; Celgene: Consultancy; Astrazeneca: Consultancy; Amgen: Consultancy. Berdeja:Abbvie: Research Funding; BMS: Consultancy, Research Funding; Karyopharm: Consultancy; Vivolux: Research Funding; Genentech, Inc.: Research Funding; Bluebird: Research Funding; Lilly: Research Funding; Constellation: Research Funding; Glenmark: Research Funding; Janssen: Consultancy, Research Funding; Legend: Consultancy; Novartis: Research Funding; Poseida: Research Funding; Prothena: Consultancy; Teva: Research Funding; Servier: Consultancy; Takeda: Consultancy, Research Funding; Bioclinica: Consultancy; EMD Sorono: Research Funding; Acetylon: Research Funding; Celgene: Consultancy, Research Funding; Cellularity: Research Funding; Kesios: Research Funding; CURIS: Research Funding; Kite Pharma: Consultancy; CRISPR Therapeutics: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Siegel:Karyopharma: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Merck: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Celulatiry: Consultancy. Jagannath:Janssen: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Legend Biotech: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Madduri:Legend: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Speaking Engagement, Speakers Bureau; Celgene: Consultancy, Honoraria; Kinevant: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Speaking Engagement, Speakers Bureau; Foundation Medicine: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Speaking Engagement, Speakers Bureau. Liedtke:Pfizer: Honoraria; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Caelum: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees. Rosenblatt:Celgene: Research Funding. Maus:arcellx: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; kite: Consultancy, Research Funding. Massaro:bluebird, bio: Current Employment, Current equity holder in publicly-traded company. Petrocca:bluebird, bio: Current Employment, Current equity holder in publicly-traded company. Caia:Celgene a BMS company: Current Employment, Current equity holder in publicly-traded company. Yang:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Celgene: Ended employment in the past 24 months. Campbell:BMS: Current Employment, Current equity holder in publicly-traded company. Hege:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Patents & Royalties: numerous, Research Funding; Celgene (acquired by Bristol Myers Squibb): Ended employment in the past 24 months; Mersana Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Arcus Biosciences (Former Board of Directors): Divested equity in a private or publicly-traded company in the past 24 months. Munshi:BMS: Consultancy; OncoPep: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; AbbVie: Consultancy; Karyopharm: Consultancy; Takeda: Consultancy; Adaptive: Consultancy; Janssen: Consultancy; C4: Current equity holder in private company; Amgen: Consultancy; Legend: Consultancy. Kochenderfer:Celgene: Patents & Royalties, Research Funding; bluebird, bio: Patents & Royalties; Kite, a Gilead company: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal